Looking Into Tonix Pharmaceuticals Before Phase 2b Fibromyalgia Results
Tonix Pharamceuticals (TNXP) is a pharmaceutical company developing drugs for the central nervous system -- CNS. The company has targeted diseases in the CNS space that have an unmet medical need. The company's main flagship product is a drug compound known as TNX-102 SL which is being tested in patients with Fibromyalgia. An upcoming catalyst in Q4 2014 may provide investors with excellent share price appreciation with positive phase 2 results.
Fibromyalgia.......
One such disease with a big unmet medical need is known as Fibromyalgia -- FM. Fibromyalgia is a syndrome where a person experiences chronic muscle pain. This disease mainly targets the muscles and soft tissue of the person's body. There are around 3 million to 6 million people in the U.S. that suffer from Fibromyalgia and current treatment options do nothing to help with the patients' quality of life. Although that is not the entire problem with having the Fibromyalgia disease. This disease also causes a multitude of other problems such as:
- Fatigue
- Sleep problems
- Painful tender points
- painful trigger points
Phase 1 Results.......
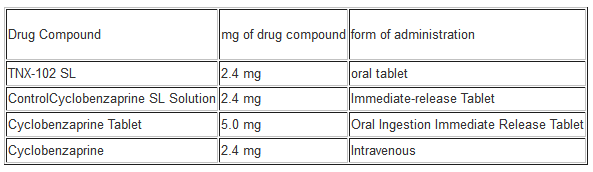
The company has already completed an initial phase 1 trial for the treatment of fibromyalgia with a drug compound known as TNX-102 SL or cyclobenzaprine. Tonix Pharmaceuticals is developing this compound as a lead compound for fibromyalgia. This means that upon successful results TNX-102 SL will be used as a first-in-class medication for these patients with fibromyalgia. This initial clinical trial tested out the various forms of administration for a cyclobenzaprine product shown below
There were approximately 23 healthy adult volunteers enrolled in this trial to determine the effect of TNX-102 SL against other forms of administration utilizing the same type of drug compound cyclobenzaprine. The results in terms of safety were great because TNX-102 SL was well tolerated and there were no adverse events reported in the patients that took the drug compound. The pharmacokinetic profile or PK profile showed that TNX-102 SL displayed better uptake and systemic circulation than all the other cyclobenzaprine products shown in the chart above. One key thing to note is that the TNX-102 SL formulation is unique because even if the cyclobenzaprine drug compound is crushed into smaller pieces it still comes nowhere near the efficiency of TNX-102 SL itself in terms of systemic circulation. Therefore these initial results substantiate that TNX-102 SL is better suited for the job than other cyclobenzaprine products utilizing other forms of administration.
Phase 2a Results....
Tonix Pharmaceuticals also noted some great positive phase 2a results in that TNX-102 SL was able to help patients improve in many different areas in terms of quality of life. Tonix Pharmaceuticals believes that by improving the sleep quality in patients with Fibromyalgia they can adequately help patients improve their quality of life throughout the day. By primarily improving the patient's sleep quality they are able to correlate it with a reduction of the fatigue symptom in Fibromyalgia. That is they have proven in this phase 2a clinical trial that improved sleep leads to a reduction in fatigue in these Fibromyalgia patients. This clinical finding can be quoted by the lead investigator of the study Dr. Harvey Modolskey who recruited and tested 36 individuals with Fibromyalgia using TNX-102 SL:
"Current treatments for FMS are inadequate, and there is substantial interest in the impact of improving sleep quality in these patients. Our analysis demonstrates improvements in FMS fatigue can be correlated with a therapeutic modality that normalizes nonrestorative sleep, and we believe this provides additional confirmation of the central importance of poor sleep quality in FMS"
So just how much did the TNX-102 SL compound help sleep quality in these patients with Fibromyalgia? Well the measure of the study used a device known as an electroencephalogram -- EEG. This EEG essentially measures the neurons of the brain and can determine the amount of activity within the brain. This EEG device uses rates of CAP A2 and A3 that both measure the amount of sleep instability within the patient. The results were positive because there was a decrease of P.M. fatigue seen in patients taking TNX-102 SL. That means that the drug was able to decrease both the CAP A2 and A3 rates. This trial reached statistical significance because the p-value of patients obtaining a decrease of CAP A2 and A3 was equal to p = 0.0064. Any p-value below .01 normally indicates a significant p value for the trial. On the other hand the placebo had no decrease effect on CAP A2 and A3 rates at all for p.m. fatigue.
Financials...
According to the 10-Q SEC filing Tonix Pharmaceuticals has cash and cash equivalents of $42.4 million dollars. This is because Tonix recently sold some shares of its common stock and was able to generate $40.7 million dollars worth of cash. The company now believes it has enough cash to run its clinical trials over the next 12 months. One thing to be aware of though is that there is a possibility that the company may dilute additional shares over the next few months. If not in 2014 then investors should at least expect some type of dilution during the year of 2015. Although all this dilution can possibly be avoided if the company is able to establish a partnership with a big pharmaceutical company that offers an upfront payment for the TNX-102 SL compound.
Risks....
As with many other biotechnology companies there are certain risks investing in these speculative type stocks:
- Successful results in phase 2 for Firbromyalgia drug may not indicate with certainty that there will be positive phase 3 results
- The phase 2 trial could come out negative in which case investors need to be aware they may lose their entire investment
- Successful results in phase 2 for the Fibromyalgia drug doesn't mean that the same drug will be positive in another indication PTSD.
- The stock may trade sporadically until the phase 2 results are to be released for the Fibromyalgia indication therefore short term the stock may decline.
- Company may have to dilute shareholders in the future if they are unable to establish a partnership for their lead Fibromyalgia drug within the next 12 months
Conclusion....
We believe that Tonix Pharmaceuticals is a good investment before the trial results are read out in early Q4 2014. Previous clinical results have validated that TNX-102 SL should garner some type of positive efficacy in its current phase 2b trial. We say this because as with all other small-cap biotechnology stocks there is no guarantee of trial success. Since we have seen excellent results in the pre-clinical setting and in the phase 1 setting then obtaining negative efficacy in Fibromyalgia is less likely to occur in the phase 2 clinical trial.
I have no position in any stocks mentioned.



