The Final Straw For Mallinckrodt?
When it rains it pours for Mallinckrodt (MNK). Last month a Delaware judge invalidated 11 patents pursuant to Inomax, one of the company's top-selling treatments; it could pave the way for Praxair (PX) to offer a generic version. The Journal of the American Medical Association ("JAMA") followed that up by questioning the effectiveness of Acthar for certain indications:
Over the past five years, why has the US government spent $1 billion on a drug that is no more effective than alternatives that are tens of thousands of dollars cheaper per treatment?
The drug is called Acthar, and for the past year it has been the focus of a study by the Oregon Health and Science University School of Medicine and Oregon State that has been trying to understand why doctors keep prescribing it for ailments it has never been proved to treat effectively.
At 39% of total revenue, Acthar is Mallinckrodt's largest-selling product. Acthar and Inomax represent a combined 54% of revenue, so over half the company's revenue could have question marks surrounding it.
Did JAMA Damage The "Acthar Effectiveness" Argument?
Acthar treats infantile spasms and certain autoimmune conditions. Its Q2 revenue grew 7% Y/Y, compared to a 5% decline for the entire company. Mallinckrodt grew its operations through acquisitions; in an about face it recently began to divest properties in to order to pare its $5.9 billion debt load. Over the past year the company engaged in asset sales of over $800 million. It is now more dependent upon Acthar whose revenue was only 31% of the company's total in the second half of 2016.
In 2015 Citron's Andrew Left questioned the effectiveness of Acthar and contended Mallinckrodt could have problems with reimbursements from insurance companies. Management is currently providing studies to prove Acthar's effectiveness, yet no conclusions have been reached:
We were also pleased that four new Acthar papers were published in the last several months, which we believe provide additional evidence for payers and prescribers to support the positioning of the product, primarily for refractory patients. Three of the publications report on clinical experience for Acthar in key promoted indications: rheumatoid arthritis, nephrotic syndrome and sarcoidosis, with clinical studies in these patient populations providing further evidence to support the treatment of refractory patients who can benefit from Acthar.
The fourth is a recently published article summarizing the extensive data contained in our Academy of Managed Care Pharmacy, or AMCP, dossier for Acthar ... Primarily used with managed care organizations, the dossier contains data on proper use for appropriate patients and the health economic benefits of the drug.
Some could potentially discard the comments of Citron's Left; as a short seller he could be viewed as biased or as having an economic incentive to criticize Acthar. However, criticism from a respected medical journal like JAMA could be seen as more objective. JAMA suggests there is a lack of quality evidence that Achar is effective for certain conditions:
But JAMA Internal Medicine notes that this goes well beyond Mallinckrodt's intentions and the impact on its shareholders. Here's how Shakil put it in the editorial (referring to Acthar as rACTH):
"The lack of high-quality evidence supporting Acthar gel's benefit in a variety of conditions, along with the unconscionable price increase by the manufacturer, should give pause to all practitioners. Not only should clinicians consider the lack of evidence supporting the efficacy of rACTH, but its story should cause us to reexamine and strengthen our standards for FDA approval, Medicare and private insurance coverage, and professional use patterns."
A large percentage of Acthar's sales comes from Medicaid/Medicare. In the past Citron has claimed up to 60% of Achar's sales has been derived from Medicaid/Medicare, while the company has quoted a figure of around 25%. If Acthar's effectiveness or pricing gains government scrutiny then Medicaid could cut back on purchases of the drug. This remains a major risk for MNK, and the JAMA study could add to that risk.
Do Doctors Have Financial Incentives To Prescribe Acthar?
Acthar is one of the most expensive drugs reimbursed by Medicaid. Per Business Insider from 2001 to today the price of the drug increased from $748 per vial to $40,000 - that represents a 28% compound annual growth rate ("CAGR"). Most of those price increases came after Questor acquired the drug in 2001. Mallinckrodt continued price increases after it acquired Questor in 2014.
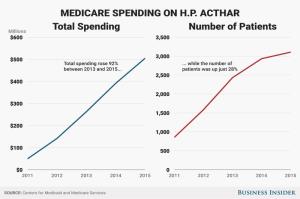
Of note is that from 2013 to 2015 total Medicare spending on Acthar increased 92%, while the number of patients increased 28%. The spike in the price also coincided with a spike in the number of prescriptions.
According to JAMA the spike in government spending on Acthar was driven by prescriptions from a small group of doctors:
The study found that a surge in government spending — ultimately a tenfold increase — on the drug was driven in part by a relatively small group of doctors who were prescribing it heavily (both with more prescriptions per person and with a spike in people being treated with it). And, the study notes, this can't just be explained away by the fact that it may be used on more "severely ill" patients.
Why would such a small number of doctors write enough prescriptions to help spike government spending on Acthar tenfold? In 2015 Medicare Part D - a program for the elderly - spent $500 million on Acthar, though the drug was specifically indicated for infants.
It might take a concerted effort by government officials before Acthar prescriptions are reduced for Medicare/Medicaid patients, if at all. A case study could be the opioid crisis. According to The Economist, between 1991 and 2011, opioid prescriptions supplied by retail pharmacies increased from 76 million to 219 million. Data suggests the proliferation of opioid use coincided with the increase in prescriptions. Now the government is intentionally trying to tamp down the number of opioid prescriptions. If governmental efforts work then it could become a blueprint to help reduce runaway prescriptions of other drugs as well.



