The Bright Future Of Pluristem Therapeutics
Pluristem Therapeutics (PSTI) is a clinical-stage biotechnology company that uses placental cells and a unique, proprietary, three-dimensional technology platform to develop cell therapies for a broad spectrum of diseases. Pluristem’s technology is based on the science of converting human placental cells into PLacental eXpanded (PLX) cells, which will respond to signals produced by damaged tissues by secreting therapeutic proteins. Pluristem is currently researching therapies for multiple indications involving inflammation and ischemia including peripheral artery disease, muscle injury, preeclampsia, and graft versus host disease.

Pluristem therapeutics manufactures their cells in-house, in state of the art facilities which allows them to produce commercial quantities of clinical-grade PLX cells, efficiently. Pluristem is unique because they are the only company to use a 3D bioreactor to manufacture placental cells. On February 14, I Know First’s Risk- Conscious forecast for aggressive stocks showed a bullish signal for PSTI. During the forecasted period the stock increased from $0.81 to $1.29.

Pluristem began 2016 in a decline, falling from it’s 3-month high of $1.55 on December 4th2015, to a 3-month low of $0.71 on January 20th 2016. At the end of 2015 the FDA granted Pluristem an orphan drug designation to their PLX-PAD cells for the treatment of Severe Preeclampsia, a disorder that results in abnormally high blood pressure during pregnancies and can affect 6-8% of pregnant women. On January 6th, the very respected peer reviewed journalClinical Science published an article, describing research performed by independent scientists at Texas A&M Health Science Center that demonstrated how the PLX-PAD cells work as therapy for Preeclampsia and also indicated that the PLacental eXpanded cells that pluristem manufactures are clinically superior to other cells. On January 14th, PSTI got FDA clearance to begin a phase 1 trial on PLX-R18 cells for the treating incomplete hematopoietic recovery following hematopoietic cell transplantation.
During the 3-day period forecasted on February 14, the National Institutes of Health began to initiate dose evaluation studies for PLX-R18 cells in Animal Studies. This event indicated that PSTI is moving forward with bringing their technology to market and gave investors reason to believe that the company will be able to make big things happen in the future, which can explain the surge in price this past week.
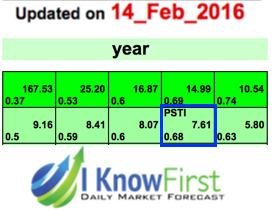
I know First is currently bullish on PSTI for the next year.
Disclosure: This article originally appeared on I Know First, a financial services firm that ...
more


