Northwest Biotherapeutics: A Variety Of Possibilities With The Future Of Immunotherapy Treatments
Northwest Biotherapeutics (NASDAQ:NWBO) is a biotechnology company developing vaccines against various types of cancer. There are many bearish articles about Northwest Biothereapeutics that point to misconceptions about the clinical trials being failures. What these bearish articles don't actually do is go into the factual trial data. Instead they talk about other schemes or plots that have nothing to do with the company itself. We are going to accomplish two things in this article that will set the record straight for skeptics and critics alike: We will take a look into the technology of Northwest Biotherapeutics and then we will discuss the current clinical trial data seen to date that validates the technology platform.
DCVAx Platform Technology
For starters, Northwest Biotherapeutics has developed a technology platform known as DCVax. What DCVax does is educate the immune system -- dendritic cells-- to go out in the body and attack particular cancerous cells. What sets DCVax apart from other immunotherapy vaccines is that the dendritic cells, once educated, are able to mobilize all antibodies in the immune system against the cancer itself. Other cancer drugs only activate one particular type of antibody as a single killing agent but are unable to activate other parts of the immune system like T-cells and dendritic cells. With DCVax the company can use all mechanisms of action of the immune system to fight the cancerous cells like one large army.
Cancerous cells normally are able to escape and replicate quicker than they are killed off, known as "escape variants". Other standard of care drugs target a single biomarker of the cancer profile, but in most cases they don't kill off the cancer completely. The cancer cells escape -- escape variant -- and are able to replicate. DCVax does something different; it targets all biomarkers of the patient's profile at the same time. Remember, each cancer patient has a unique cancer cell profile. By targeting all biomarkers at once, DCVax greatly reduces the chance of these cancerous cells being able to perform an "escape variant." But the company has not stopped there; they realize that some cancers can form into solid tumors and are addressing that issue as well.
Northwest Biotherapeutics has formed two distinct subcategories for its DCVAx technology they are:
- DCVax-L
- DCVax-Direct
DCVax-L
In both DCVax-L and DVax-Direct the company first does a blood draw on the patient taking his (or her) own specific immune system cells --monocytes. These monocytes are infused with biomarkers known as "antigens" that are the basis for combating the cancer itself. The purpose of putting these antigens on the monocyte cells is so that the main immune system dendritic cell can obtain the knowledge it learns from the antigens and pass it down to the rest of the immune system. The new dendtritic cells are then infused back into the body like a "flu shot" with a needle and achieve their destructive function against cancerous cells with the similar biomarker.
DCVax-Direct
The process for DCVax-Direct is the same as DCVax-L except for the fact that in DCVax-Direct the antigens are injected directly into the solid tumor of the patient, where the dendritic cell process begins. Once again this is where the dendritic cells in the tumor convey the antigens to the rest of the immune system that go out and target other similar cancerous cells.
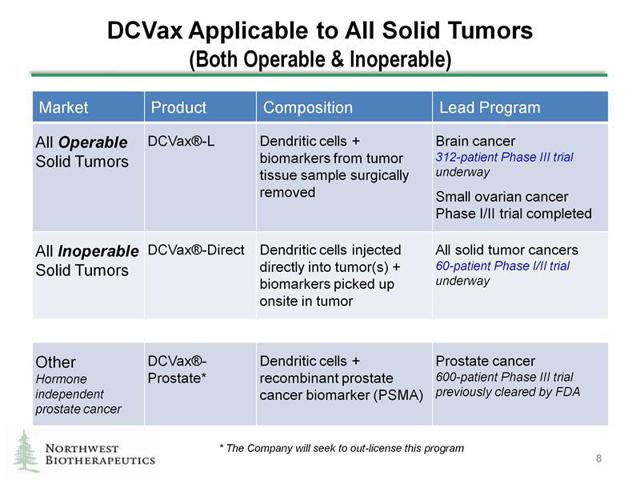
DCVax-L and DCVax-Direct can both be utilized for Operable and Inoperable tumors as shown in the graphic below:
Source:Company Data
Dendritic Cell Process
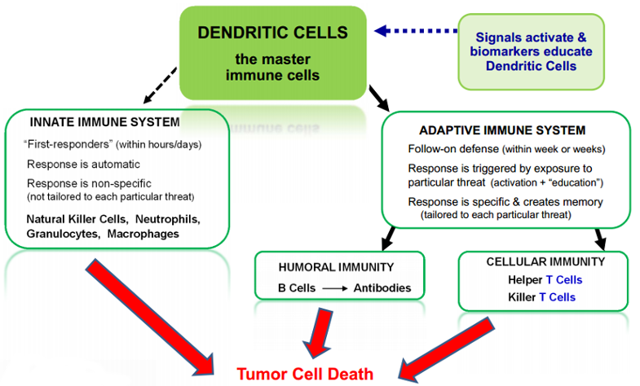
As we have mentioned above, Dendritic Cells motivate the entire immune system to attack key targets -- cancerous cells. Think about the Dendritic cells as the leader of the pack of the immune system where they educate and direct what T-cells, macrophages, and B-cells all should target and destroy. Some of the description on the dendritic cell process can be seen below.
source: www.nwbio.com/dendritic-cell-immunotherapy/
As you can see in the graphic above, the Dendritic cell acts as the main leader of the entire immune system process. Northwest Biotherapeutics believes that by giving the dendritic cell specific antigen instructions, that cell will give the message to all other immune system components, thereby increasing the probability of the treatment working for that specific patient. The company has been working on its lead candidate trial for a type of cancer known as Glioblastoma Multiforme.
The Dangerous Glioblastoma Multiforme
Glioblastoma is a type of brain cancer that forms on the tissue of the brain. The tissue of the brain is composed of cells that are known as astrocytes. The tumors grow from these astrocytes and can cause serious problems for the brain itself. One thing to note though is that these tumors can also grow on the patients' spinal cord as well and lead to other types of problems.
Glioblastoma is dangerous in itself but Northwest is targeting an even more aggressive form known as Glioblastoma multiforme. This means that the Glioblastoma has made it to grade 4 and is the hardest form to treat. Glioblastoma multiforme has no effective therapies currently and today's standard of care treatments do nothing to help patients with this type of cancer. Surgery is ineffective because the cancer topographically diffuses into various areas of the brain, making any attempt to remove the cancer impossible. Chemotherapy and other radiation therapy does nothing to stop the cancer because Glioblastoma is "multiforme" in nature implying that it carries a variety of possibilities in mutation. This means it is difficult to find a treatment that will target all aspects of the tumor itself and not just one component of the tumor.
This is where DCVax-L can come in and combat the cancer with all available biomarkers loaded onto the monocytes mentioned above. As we described above, the monocytes act as the leader and pass down information to other parts of the immune system. This means that DCVax-L can use all parts of the immune system to attack as a unit, all at once. DCVax-L, targeting multiple biomarkers in one fell swoop, eliminates the possibility for the tumor to perform an escape variant as mentioned above, making clinical outcome success a great possibility.
DCVax-L Trials for Glioblastoma Multiforme phase 3
Currently DCVax-L is in a phase 3 trial treating patients with Glioblastoma multiforme -- GBM. The current phase 3 trial is estimated to enroll up to 348 patients across 51 sites in the United States. The phase 3 trial for DCVax-L is being run as a double-blind, randomized, placebo controlled trial. Many bearish articles portray this trial as if the management of Northwest Biotherapeutics can see the data and know that the trial is a complete failure. This is totally false as anyone that works in the pharmaceutical industry knows what the term "double-blind" in a clinical trial means. Double-blind means that the company or principal investigators of the trials don't know which patients receive DCVax-L and which ones receive placebo until the trial is un-blinded at the finish. So how can management know the results of the phase 3 trial, as many of these bearish articles claim? There is no way they can know, because not even the principal investigators themselves that are running these clinical trials at these sites know which patient receives which drug. The primary endpoint of the phase 3 clinical trial is testing for Progression-Free Survival -- PFS. This means the trial is attempting to see how well it does with patients continuing treatment with DCVax-L without progressing further with their cancer. The Secondary endpoint is assessing other measures like overall survival, which measures how long patients survive with treatment of DCVax-L over a period of time.
DCVax-L Compassionate Use Trial
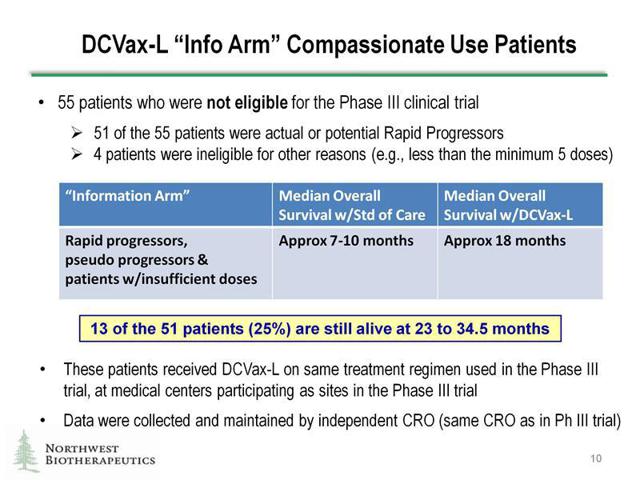
The company had recently reported results from an additional trial it ran separately from its phase 3 clinical trial currently underway in patients with GBM. The difference is that these patients didn't qualify for the DCVax-L phase 3 trial because they were considered "rapid progressors". These are patients whose GBM recurrence occurs in only a matter of six weeks even with standard of care therapies -- radiation therapy and tumor removal by surgery. This separate trial being run is known as the "information arm" trial because it is following these rapid progressor patients separately from the main phase 3 trial. These rapid progressors in this information arm trial are receiving the same form of treatment, DCVax-L. These patients who have such a quick tumor recurrence still have shown to benefit greatly from receiving the DCVax-L treatment.
To give a snapshot idea of the difference between GBM patients and those GBM patients who are rapid progressors, we can look at typical survival utilizing standard of care treatments options. GBM patients have a median survival time of at least 14.6 months versus GBM rapid progressor patients who only have a median survival of 7 to 10 months. Also, rapid progressor GBM patients don't typically respond well to any type of treatment given, so the fact that they responded so well taking DCVax-L shows that this treatment from Northwest is very promising. Not only that but the numbers speak for themselves in this information arm trial. The median overall survival for these 55 patients was 18 months. That is pretty good considering median survival for these rapid progressors is typically only 7 to 10 months.
Source: Company Data
In addition the most recent update shows that 13 of the 51 patients are still alive at 23 to 34.5 months as shown above. This data was also kept confidential so management had no access to this data whatsoever. The data was collected and analyzed by an independent body known as a CRO (contract research organization).
DCVax-L in Phase I/II Study Results
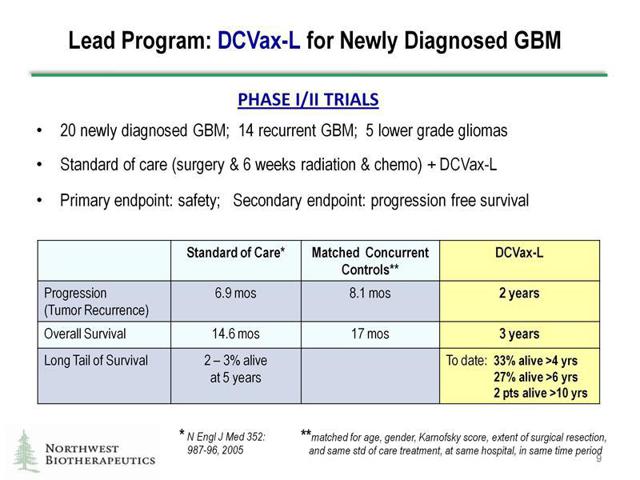
The DCVax-L trial is currently underway in phase 3. Key evidence, shown above, in a separate trial under compassionate use allowed us to see that DCVax-L is producing good clinical results. But to keep people's fears in check we can also take a look into additional results from a prior clinical trial using DCVax-L. These previous results in a phase I/II study, along with the compassionate use results, should allay investors' fears that the phase 3 clinical trial will fail to improve clinical outcome in patients with Glioblastoma.
Source: Company Data
In the phase I/II trial shown above, 20 patients were recruited into the clinical trial to determine if DCVax-L along with placebo could help improve PFS -- progression-free survival-- in patients with Glioblastoma Multiforme. One thing to note above is that two patients taking DCVax-L were alive greater than 10 years cancer free. That is pretty good considering average survival time for these patients using placebo is typically only 14 months. The results above speak for themselves: a huge percentage of additional patients have progression-free survival times past 4 years and 6 years respectively. How someone can take the bearish side of these clinical results, and claim that maybe it was a miracle these patients survived that long, is kind of hard to fathom. Any doctor or informed investors can see that these DCVax-L results are outstanding compared to any placebo treatment given. One of the patients in this trial was diagnosed with GBM back in 2003 and doctors only gave him two months to live. By taking DCVax-L the patient was able to survive cancer free past the 10 year mark to 2013, which is unheard of according to CEO of NorthWest Biotherapeutics Linda Powers:
"With 'State of the art' treatments today, 10-year survival in GBM is unheard of. Indeed, even survival half that long is virtually unheard of. The 'long tail' of survival among a large percentage of our patients is a particularly compelling aspect of our DCVax technology."
That one patient is now cancer free and is doing well with his family.
Germany Gets Early Access For DCVax-L
Earlier in 2014 German regulatory officials announced that they had given DCVax-L 'Hospital Exemption approval' under German Drug Law. The DCVax-L vaccine is the first immunotherapy treatment to be approved in Germany for this Hospital Exemption. On October 14, 2014 Northwest Biotherapeutics announced that the first German patient has been treated with DCVax-L and many other patients are wanting to be included on a waiting list to receive treatment with DCVax as well. This form of treatment is most welcome, as mentioned in a quote by Prof. Dr. Dietmar Krex, who is senior neurosurgeon at the University Hospital of Dresden, Germany:
"The Hospital Exemption for DCVax-L is a groundbreaking step, giving hope and offering a new treatment modality to patients with the disastrous diagnosis of a malignant glial brain tumor. Of course, we are also looking forward to completing the Phase III trial as soon as possible to confirm the effectiveness of the DCVax-L treatment."
It is good to see that Germany has allowed DCVax-L to be given to these patients with GBM as a treatment like this could prolong their lives.
UK Wants Early Access For DCVax-L As Well
One country's validated interest in early access to a vaccine that has yet to be approved is significant, but that's not all. On September 2014 the UK Government granted its first UK 'Promising Innovative Medicine' award, to DCVax-L The UK stated that this is the first step for its Early Access to Medicine Scheme -- EAMS. This first validation was welcomed by Life Sciences Minister George Freeman in this quote:
"Making Britain the best place in the world for science, research and development is a central part of our long term economic plan. We want to make Britain the best place in the world to design and deliver 21st Century healthcare technologies which is central to our life science strategy. This Promising Innovative Medicine designation is the first crucial step in accelerating access to new medicines, giving real hope to patients and their families."
There are three takeaways from the quote above. For starters, note that the UK Life Science Minister sees DCVax-L as a 21st century therapy that is making a huge breakthrough in the biotechnology industry. Secondly, the EAMS was established back in April of 2014 and Northwest was awarded this designation just recently in September 2014. This indicates that the UK regulatory authority doesn't just hand it out without deliberation; the treatment in question must literally be transformational in the science industry. The final takeaway from the Minister's quote is that DCVax-L brings hope to patients and their families, as evidenced by some of the prolonged-life results discussed earlier.
Diversified Possibilities In Cancer Immunotherapy
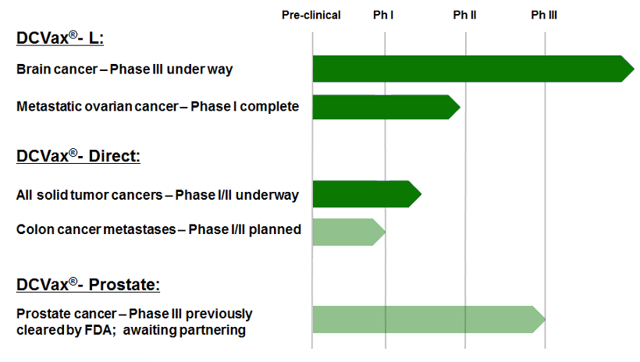
DCVax-L in Glioblastoma Multiforme is just one of the possibilities for NorthWest Biotherapeutics. The company has seen great results to date in other cancers besides Glioblastoma Multiforme. Utilizing the DCVax technology platform Northwest Biothereapeutics is expanding its pipeline to a multitude of other cancer targets shown in the graphic below.
(click to enlarge)
Source: www.nwbio.com/clinical-trials/
Validation from the phase 3 compassionate use trial results from the 55 patients, UK early use, and Germany early use pretty much trump the bearish belief that DCVax is a failure. But we will not stop there; we will further show that the DCVax platform works well in many different targets and put the bearish argument to rest once and for all. Let's accomplish this by further validating the DCVax platform technology against solid tumor cancers.
DCVax-Direct Against Solid Tumors
The company reported early results for DCVax-Direct in 2014 as well establishing proof of concept that DCVax can be utilized against solid tumor cancers. This trial recruited 9 patients to analyze DCVax-Direct being able to help reduce these advanced tumors. All 9 patients, who received only 4 injections out of the 6 total injections needed to complete the dosing treatment, displayed tumor cell death, tumor shrinkage, and immune cell accumulation in their tumors. If we analyze this initial data we can conclude that the immune cell accumulation confirms what we described above with the DCVax technology in that DCVax is able to educate the immune system to immediately target where the tumor cells reside. In addition to these findings, 3 out of the 9 patients had biopsies performed on their solid tumors. The biopsies showed that no live tumor cells were found on the solid tumors of those 3 patients. This falls in line with the notion of DCVax being able to not only educate the immune system to achieve Progression-Free Survival, but get rid of the tumor cells completely and improve overall survival. If that wasn't enough, these 3 patients who had completely gotten rid of their tumor cells were in metastatic state. That is, that each of these patients had a tumor in the worst state possible and normally at that point not even standard of care treatment -- surgery and radiation -- offers any type of hope. This can be evidenced by the quote from the CEO of Northwest Biotherapeutics Linda Powers:
"These early glimpses are indicating an increasingly encouraging picture especially the absence of any liver tumor cells in 3 of the patients who have received 4 of the 6 planned injections of DCVax-Direct"
The key takeaway from the quote above is that these metastatic cancer patients with no treatment options only needed 4 of the 6 planned injections to kill tumor cells. This is substantial because if a few patients are able to kill off their tumor cells after only 4 injections than that means by 6 injections the rest of the patients should theoretically achieve greater clinical success. This also proves that the DCVax Platform has thus once again been validated in an additional mid-stage clinical trial.
Financials
Northwest Biotherapeutics recently raised $11.5 million of financing by selling shares to an existing single shareholder entity of the company. The shares of Northwest Biotherapeutics were sold at $5.05 per share. This $5.05 per share price point sold is slightly above the 52-week low of $3.10 per share which gives the stock a kind of cushion. According to the 10-Q SEC filing Northwest had $12.4 million of cash as of June 30, 2014. If we add the $12.4 million along with the recent raise of cash a few weeks ago of $11.5 million dollars that nets the company approximately $23.9 million dollars of operating cash. This should last the company around a one year time frame but that means that further dilution will be needed once again before the cash runs out.
Risks
There are many types of risks associated with biotechnology stocks that should be considered before investing in one of these speculative companies:
- The final phase 3 results may yield sufficient efficacy for approval but may or may not fare better than future treatments from other biotechnology companies
- The final phase 3 results may meet on the secondary endpoint for Overall survival but fall short in the primary endpoint of Progression-Free Survival
- Overall Weakness in the biotechnology index may fluctuate the share price lower in the coming months.
- Even upon successful phase 3 results there is no guarantee that the share price will rise by a higher percentage accordingly
- Future bearish articles may try to claim unsubstantiated facts as truth, thereby sending the share price lower in the short-term.Therefore the share price may feel short-term volatility
- Success in the phase 3 trial for Glioblastoma won't mean automatic success in the solid tumors trial for DCVax
- There is a possibility for further dilution later on as the company may need to raise more cash for additional clinical trials utilizing DCVax
- Additional problems with the U.S. economy, Ebola fears, and weak economies abroad may create further volatility for biotechnology stocks sending the share price lower
Conclusion
We believe that shares of Northwest Biotherapeutics are severely undervalued. We have laid out our bullish thesis against many bearish articles that claim DCVax is a failure. We have given a multitude of evidence proving that DCVax is not a failure and in fact has helped to prolong the lives of many patients. With the current share price at around $4.84 per share we believe that Northwest Biotherapeutics is at a great buying opportunity for the long-term.
Disclosure: Long on NWBO.