Gilead Is Persona Non Grata
Gilead (GILD) is down over 3% since earnings were released. Heading into earnings I warned that revenue could fall sequentially by 11%. I also warned about Gilead heading into Q2 earnings, yet the company received a jolt from patient starts as more baby boomers received HCV screenings and diagnoses.
Total Q3 revenue fell 9%, while HCV revenue was off an eye-popping 28%. We have known that the decline in the HCV runway would accelerate. On the Q2 earnings call Gilead's management implied HCV revenue could fall by about 24% in the second half of the year. Nonetheless, I was even taken aback by the 28% drop off.
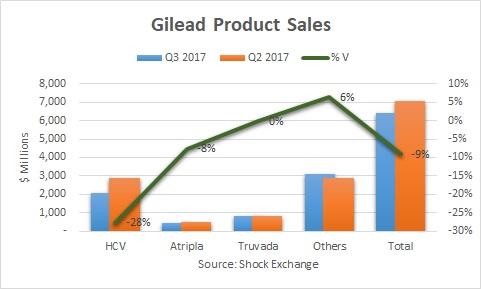
Lower patient starts and increased competition hurt Gilead's HCV franchise this quarter. U.S. HCV revenue was off 26% sequentially, which implies the decline internationally was even more pronounced. Revenue from Epclusa fell 25% Q/Q. This was a sea change from Q2 when Epclusa bounced 31%. On a dollar basis Epclusa's revenue was $882 million; it effectively lost all its momentum from Q1 when it generated $892 million. AbbVie (ABBV) launched its HCV regimen Mavyret this quarter. The drug is indicated to treat genotypes 2-6, which could pose a threat to Epclusa. It generated $100 million in revenue in Q3, and could potentially be the reason for the sharp pull back in Epclusa sales. If that is the case then another catalyst for Gilead might be under pressure.
Non-HCV revenue was up 4% sequentially. Viread and Truvada lost exclusivity. Truvada was flat while Viread was off 9%. Both could fall further as generics competition kicks in. Gilead received the benefit of a $332 million favorable adjustment to a rebate reserve that increased antiviral revenues. Overall, it was a noisy quarter for the segment. I am curious how Gilead will make up for the loss of exclusivity for Viread and Truvada going forward.
All About Kite
GILD trades at under 6x EBITDA. The company is also a cash cow. The market does not place much value on Gilead's $41 billion cash hoard given the low interest rate environment. It is hard to give it much credit for its recent $12 billion acquisition of Kite since Kite does not generate earnings yet. The transaction was a masterstroke. Kite received FDA approval for Yescarta, which treats B-cell non-Hodgkin lymphoma, within months of the deal.
Cowen intimates as many as 5,300 patients relapsed/refractory patients will be good candidates. It would take a while for the company to be able to treat that many patients, however. Gilead will likely plan staged roll outs of Yescarta. It could potentially treat over 1,000 next year and build up to 5,000 over time. 5,300 patients at a full price of $373,000 per regimen would only generate about $2 billion in annual revenue. That pales in comparison to the HCV franchise which has a run-rate of $8 billion in revenue, albeit in rapid decline.
Yescarta appears to be the next growth engine for Gilead. Moreover, the company might have hung its hat on being an acquirer of late-stage biotech companies. R&D was $789 million, down from $864 million in the previous quarter. R&D has consistently been 12% to 14% of revenue in the past few quarters. However, on a dollar basis it has fallen each quarter since Q2 2016 when it was $1.5 billion. That is a huge contrast with Celgene (CELG) who increased R&D Q/Q despite giving weak revenue guidance. Changes in R&D spending likely gives an indication on where each company thinks future growth will come from.



